The Autumn Crocus Flower’s Colchicine Molecule Acts as a Cancer Mitotic-tubulin Inhibitor and a Vascular Disturbing Agent (VDA): the British & French Contributions
In this blog, colchicine, one of the active molecules that comes from the crocus flower, known as the pink “naked lady”, will be examined for its cancer control and reversal potential. (Section A). Thereafter, colchicine will be looked at from the cancer prevention viewpoint. (Section B).
 TOP: Scientific Name: Colchicum autamanale.Other Common Names: Meadow Saffron, Autumn Crocus, Naked Lady. Pharmaceutically, the bulb-like corms of Colchicum autumnale (crocus) contain colchicine, a molecule that has a narrow therapeutic index (ie, the US FDA and the European EMA have only approved it for the treatment of gout and familial Mediterranean fever). Research and clinical trials are examaning its usefulness for cancer control and reversal.
TOP: Scientific Name: Colchicum autamanale.Other Common Names: Meadow Saffron, Autumn Crocus, Naked Lady. Pharmaceutically, the bulb-like corms of Colchicum autumnale (crocus) contain colchicine, a molecule that has a narrow therapeutic index (ie, the US FDA and the European EMA have only approved it for the treatment of gout and familial Mediterranean fever). Research and clinical trials are examaning its usefulness for cancer control and reversal.
SECTION A
COLCHICINE: A CANDIDATE MOLECULE FOR CANCER THERAPY
Over the years, the “naked lady’s” (1) colchicine has been used for different health conditions. (2) It has also been tested as an anti-cancer agent, in particular, for its anti mitotic, anti-tubulin and anti- vascularization effects against cancer cells. (3). However, colchicine can be highly toxic to non cancerous cells as well. (4) To address this hurdle, scientists at Bradford university in England have found a way to use colchicine selectively against cancer cells, by disruptiong its vascular network via different biochemical mechanisms. (5)
In this perspective, The Bradford University researchers in West Yorkshire England, led by Professor Laurence Patterson, have tweaked the colchicine molecule to make it much less toxic than what it is. They have done this by delivering colchicine directly to a tumour thereby preventing damage to the other non cancerous cells.
TARGETING THE MATRIX METALLOPROTEASES
The Bradford scientists have accomplished this “breakthrough” thanks to, inter alia, the matrix metalloproteinase (MMP) enzymes, which are abandantly produced by cancer cells. Matrix metalloproteinases comprise a family of 24 zinc-dependent endopeptidases with structural similarity. These enzymes have a pivotal role in tumorigenesis and tumor progression (6)
In order to target colchicine directly to tumours then, the researchers had to first target this family of proteins, the MMTs, if only because cancer cells produce a huge amount of these robust proteins that are used to destroy the gelatinous gloop that surrounds healthy cells, (eg the extracellular matrix). Once this gelatinous gloop is cleared, cancer cells can then start its angiogenesis mission, the building of new blood vessels to grow towards them, thereby providing essential oxygen and nutrients to cancer cells.
THE TROJAN HORSE TECHNIQUE
To exploit this above-mentinoed particularity, the Bradford researchers attached long molecular ‘tails’ to individual colchicine molecules. Once this ‘tail’ was hacked off by MMP enzymes then it was activated into the potent cell-killing form of the drug that kills cancer cells as well as new growing blood vessels thereby starving the tumor of nutrients and oxygen. (7)

Art form of the naked lady’s shapes
SECTION B
COLCHICINE FOR PREVENTION
In the 1970s, French scientist André Gernez proposed to use a protocole based colchicine as a pubilc health measure. This “active prevention” protocole, designed for risk populations (notably between 40 and 65 years of age) is based on light chemotherapeutic agents, in particular colchine and chloral in association with growth hormone and DHEA inhibition, (including via the gamma knife radiation technique), caloric restriction, and inter alia, supplementation. Dr Gernez’s carcinogenesis’ theory is that cancer cells thrive in an alkaline environment and must be preventively killed with light “chemo). Colchicine is a central element of this active protocole. (Source)
While this protocole was shown by INSERM to be relatively successful in animals, the conclusion for human use has not been fully investigated. As a result, the French authorities and official cancer organizations have deemed this protocol too dangerous and not sufficiently proven. (Source).
On the other hand, new evidence has shown that part of this Gernez protocol may be reasonable, in particular the use of low dose cholchicine as do gout patients (eg around .6 mg). (Source) One of these studies has found a significant association between reduced risk of incident cancers, particularly in prostate and colorectal cancers, and use of colchicine in male patients with gout.
This study represents the first to report a significant association between colchicine use and lower risk of incidental cancers in male gout patients, who are known to be at higher risk of developing cancers. This study, which included more than a hundred thousand male gout patients, found a significant association between colchicine use and a decreased risk of cancer after adjustment
” Colchicine use was most significantly associated with a decreased risk of prostate and colorectal cancers in our male gout patients in this study. One well-known anticancer mechanism for colchicine is the direct colchicine–tubulin interaction, which perturbs the assembly dynamics of microtubules.32–34 Fakih et al21″ (8)
Furthermore, colchicine, combined synergiscally with other compounds, in particular with estramustine, has been shown to be effective with one form of prostate cancer that is called “aggressive prostate cancer” or “hormone-refractory” prostate cancer (which continues to be associated with a poor prognosis). By inhibitig microtubule function, aggressive prostate cancer cells have gone into apoptosis and died.
“In clinically achievable concentrations, the combination of estramustine and colchicine was cytotoxic to both the Dunning rat prostate adenocarcinoma cell line MAT-LyLu (MLL) and human prostate cancer cells (PC-3).” (9)
Another study using a combinations of platinum with paclitaxel and colchicine in ovarian cancer cell lines has also shown promise. (10)
DISCUSSION
Regarding the first vascular disrupting colchine experimentations, in vitro and animal studies have been deemed impressive. But they lack human trials. Furthermore, they’re just one of several hundreds of similar intricate approaches taken to tackle cancer by researchers around the globe, which are generally referred to as ‘enzyme-prodrug therapy’, and involve activating a harmless form of a drug near or in a tumour. (11) There is no question that in conventional oncology, disruption of the vascular network within tumors is known to be an efficient chemotherapeutic strategy. (12) But we still lack long duration studies that show that these anti-cancer approaches do not coax cancer cells to come back later on. Drug resistance has always been a major hurdle in conventional oncology. The Herceptin breast cancer ‘wonder drug’ or the expensive anti-angiogenesis Avastin drugs are cases in point. Nor do we have enough studies that show that healthy cells are sufficiently immune from these chemical interventions. (13)
As for the French “active prevention” approach to colchicine, the public authorities have denied human trial funding while the alternative health sources have been unable to demonstrate long term efficiency and safety among the risk population that would be preventively targeted (from 40 to 65 years olders). Furthermore, using preventively chemotherapy (even in a light dose) on populations that have no cancer could spur ill consequences as would Dr Gernez’s preventive “gamma knife” radiation therapy that would zap permanently human growth hormones from being produced.
On the other hand, most of Dr Gernez’s recommendations on caloric restriction, oxygen, exercises, diet and supplementation are all the more reasonable that these proposals have been promoted by holistic oncology advocates for centuries. Though for different reasons than what Dr Gernez invokes. In particular, regarding the acidification-alkalization question and a few other aspects of his carcinogenesis theory, including, but not limited to his ideas on DHEA, which for him ought to be suppressed. For holistic oncology, this hormone, just like the human “growth hormone” are rejuvenation hormones to be respected and holistically encouraged.
Meanwhile, colchicine’s role in helping to prevent cetrain cancers like prostate cancer for gout patients needs to be better demonstrated. Futhermore, in holistic oncology and advanced public health policies, there are many other safer, more efficient and cost friendly options that render all of this colchicine debate moot. (See ACR institute‘s work on these questions).
CONCLUSION
Mainstream scientists and their pharmaceutical sponsors have been characterizing colchicine as a “cancer chemotherapy breakthrough” that could ‘bring hope to millions’, ‘dramatically reduce side-effects’, and ‘kill tumours in one treatment’ (14). Just this language should get our “red warning lights” to blink.
Despite a recent mainstream treatment paradigm shift based on molecularly-targeted therapies and immunotherapy, the majority of patients still receive chemotherapy during their treatment course, either used alone or in combination. Therefore, tubulin inhibitors like colchicine have their place in conventional oncology. (15) And even in integrative oncology, when they are used as Dr Gernez has proposed.
But if we desire an approach to cancer which is even more safe, more efficient, more cost friendly and ends the cancer epidemic, then holistic oncology is the way to go, at least once the public health authorities muster up enough political courage to recognize that a chemical and symptomatic approach to chronic diseases like cancer is a never-ending futulity battle, notwithstanding some improvement. Today’s prevalence of cancer is the result of both the type of society and values we have invested in and the accumulation of stale medical dogmas. To meet this twin challenge will take more time and effort.
Pr. Joubert (HOM producer)
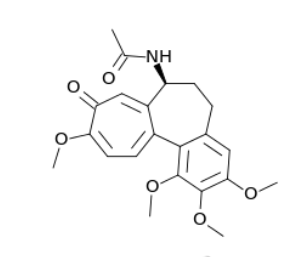
Top: Colchicine’s molecular structure
REFERENCE AND PRECISION NOTES
(1). During the fall, Naked Ladies like to make their appearances in the garden! Naked Lady is a nickname for Autumn Crocus, a popular fall-flowering bulb. The cheeky name comes from the fact that these flowers will emerge from the ground without leaves during the fall months, looking bare or naked. Autumn crocuses are easily identified by their large, showy globlet-shaped flowers blooming on bare stems. Flowers, which come in shades of pink, purple and white, are native to European and North African meadows. These fall-blooming flowers will add colour and beauty to any garden. They would would be ideal for rock gardens or among other low-growing plants. Naked ladies prefer sunny but sheltered locations, along with fertile, well-drained soils. Interestingly, while the Autumn crocus and the spring-flowering crocus may look similar, they are not related. The Autumn crocus is not a true crocus. The spring flowering crocus, which is considered a true crocus, belongs to Iridaceae, the iris family. Another difference is that the spring crocus is harmless, while the Autumn Crocus is poisonous to both animals and humans, as it contains colchincine, a deadly toxin which has been the object of medicinal use. Some bickers mistakingly eat this “naked lady” thinking it is wild garlic. This ingestion can be fatal. (Source)
(2). Colchicine was first isolated in 1820 by the French chemists P. S. Pelletier and J. B.Caventou. Colchicine prevents microtubule assembly and thereby disrupts inflammasome activation, microtubule-based inflammatory cell chemotaxis, generation of leukotrienes and cytokines, and phagocytosis. Many of these cellular processes can be found in other diseases involving chronic inflammation. The multimodal mechanism of action of colchicine suggests potential efficacy of colchicine in other comorbid conditions associated with gout, such as osteoarthritis and cardiovascular disease.Colchicine has multiple mechanisms of action that affect inflammatory processes and result in its utility for treating and preventing acute gout flare. Other chronic inflammatory diseases that invoke these molecular pathways also exist and are presently tested for new therapeutic applications. (Source)
(3). There have been many tubulin inhibitors that have been researched on, including taxanes and vinca alkaloids which have been components of chemotherapy regimens used in advanced solid cancers.. The FDA has also approved tubulin inhibitors as paclitaxel, docetaxel, vinorelbine, and nab-paclitaxel. Novel tubulin inhibitors include cabazitaxel, eribulin, ixabepilone, patupilone, plinabulin and colchicine. Cf. Opin Pharmacother. 2017 May;18(7):701-716.
(4). The naked lady’s poison can lead to cardiotoxicity, cardiac ischemia, arrhythmias and organ shut down. See above link, in the first footnote as well as the following reference. Van Heeckeren WJ, Bhakta S, Ortiz J, et al. Promise of new vascular-disrupting agents balanced with cardiac toxicity: is it time for oncologists to get to know their cardiologists? J Clin Oncol 2006;24:1485–8.
(5) Atkinson, J., Falconer, R., Edwards, D., Pennington, C., Siller, C., Shnyder, S., Bibby, M., Patterson, L., Loadman, P., & Gill, J. (2010). Development of a Novel Tumor-Targeted Vascular Disrupting Agent Activated by Membrane-Type Matrix Metalloproteinases Cancer Research, 70 (17), 6902-6912 DOI: 10.1158/0008-5472.CAN-10-1440 (Source)
(6). Originally, it was believed that the MMPs functioned purely to break down the extracellular matrix to facilitate tumor invasion, a concept now shown to be too simplistic (Cf, Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer 2002;2:161–74). In addition to extracellular matrix breakdown and tumor cell invasion, MMPs also play a major role in controlling tumor cell growth, migration, differentiation, and ultimately metastasis. Cf. Deryugina EI, Quigley JP. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev 2006;25:9–34. And: CM, Kleifeld O. Tumour microenvironment—opinion: validating matrix metalloproteinases as drug targets and anti-targets for cancer therapy. Nat Rev Cancer 2006;6:227–39.
(7). The idea is that in healthy tissues (which don’t have much MMP), the modified colchicine molecule is essentially harmless. But when it arrives in areas with lots of MMP – namely tumours – the tail is cut off and the drug is activated.
(8). Colchicine Significantly Reduces Incident Cancer in Gout Male Patients A 12-Year Cohort Study Ming-Chun Kuo, MD, Shun-Jen Chang, PhD, and Ming-Chia Hsieh, MD, PhD Monitoring Editor: Yufang Ma. Prostate. 1995 Jun;26(6):310-5.
(9) Fakih M, Yagoda A, Replogle T, et al. Inhibition of prostate cancer growth by estramustine and colchicine. Prostate 1995; 26:310–315. (Source). Colchicine severely damages a cell’s internal scaffolding by blocking the action of a protein called tubulin. Because tubulin plays a vital role in helping cells divide in two, colchicine effectively stops cell division in its tracks – an important trait for any potential cancer drug.
(10). “Binary combinations of platinum compounds Cs, Ox, YH12 and TH1 with plant compounds Tx and Co applied to ovarian cancer cell lines showed sequence- and concentration-dependent synergism. The results may have profound implications in therapy, if found to be true in vivo”. (Source)
(11). Cf. info.cancerresearchuk.org/news/archive/pressrelease/2007-05-17-suicide-gene-therapy-kills-bowel-cancer-cells. Recently, the Yorkshire Cancer Research has announced plans to fund the first clinical trial of a ‘smart-bomb’ drug discovered in Bradford, which was discovered with funding from the charity by researchers led by Professor Laurence Patterson at the University of Bradford’s Institute of Cancer Therapeutics in 2011.
(12). A good number of vascular disrupting agents (VDA) are currently undergoing clinical trials. Several VDAs target the colchicine-binding site of tubulin, induce rapid conformational changes in tumor vasculature, blood vessel occlusion, and consequent tumor necrosis. The disruption of a single tumor blood vessel has the effect of starving and consequently killing the many tumor cells it supports, making this an effective therapeutic strategy. Cf. Lippert JW III.. Vascular disrupting agents. Bioorg Med Chem 2007;15:605–15. Kanthou C, Tozer GM. Microtubule depolymerizing vascular disrupting agents: novel therapeutic agents for oncology and other pathologies. Int J Exp Pathol 2009;90:284–94. Davis PD, Dougherty GJ, Blakey DC, et al. ZD6126: a novel vascular-targeting agent that causes selective destruction of tumor vasculature. Cancer Res 2002;62:7247–53. Tozer GM, Kanthou C, Baguley BC. Disrupting tumour blood vessels. Nat Rev Cancer 2005;5:423–35.CrossRefPubMedGoogle Scholar
(13). Colchicine severely damages a cell’s internal scaffolding by blocking the action of a protein called tubulin. Because tubulin plays a vital role in helping cells divide in two, colchicine effectively stops cell division in its tracks – an important trait for any potential cancer drug. Unfortunately, colchicine doesn’t just attack cancer cells – it will stop healthy cells from dividing too. As a result, colchicine is regarded as a poison, with similar effects on the body to arsenic
(14). See the following link: www.dailymail.co.uk/health/article-2036279/Crocus-drug-kill-tumours-treatment-minimal-effects.html?ito=feeds-newsxml
(15). It also discusses new tubulin inhibitor combinations with immunotherapy (PD-1/PD-L1 inhibitors) and molecularly-targeted therapies (e.g. anti-angiogenic agents, mTOR inhibitors, heat shock protein 90 inhibitors, MEK inhibitors, and anti-HER3 agents)….Vascular disrupting agents (VDA) offer a strategy to starve solid tumors of nutrients and oxygen concomitant with tumor shrinkage. Several VDAs have progressed into early clinical trials, but their therapeutic value seems to be compromised by systemic toxicity. In
EXHIBITS
Dr Pierre Delahousse on Dr Gernez cancer prevention approach (colchicine with chloral hydrate).
[vsw id=”0Wk3KSJ4xN8″ source=”youtube” width=”325″ height=”244″ autoplay=”no”]
Dr André Gernez
[vsw id=”JVCmkhExZPI” source=”youtube” width=”325″ height=”244″ autoplay=”no”]

TOP: Image of the mitotic spindle in a human cell showing microtubules in green, chromosomes (DNA) in blue, and kinetochores in red. VDAs (vascular disrupting agents) target the colchicine-binding site of tubulins. Colchicine inhibits microtubule polymerization by binding to tubulin, one of the main constituents of microtubules. Availability of tubulin is essential to mitosis, so colchicine effectively functions as a “mitotic poison” or spindle poison
[vsw id=”N6F4VFHeyqI” source=”youtube” width=”325″ height=”244″ autoplay=”no”]
TOP: In this mycrotubule dynamics lecture, the host will explain the polymerization and depolymerization of tubulin proteins and their properties to produce the cytoskeleton system of a cell. (Source) Microtubule destabilization is the main mechanism of action in many of the best chemotherapeutic agents. Microtubules are structures within a cell that play a key role in mitosis, a crucial stage in the cell division process. Chemically, a microtubule is a gigantic biological aggregate formed by protein subunits called tubulin. An anti-cancer drug can bind to at least three distinct areas, or pockets, on the microtubule—namely the colchicine site, the vinca alkaloid site, and the taxol site etc.

TOP: Colchicum autumnale, a cytotoxic “chemo”, commonly known as autumn crocus, meadow saffron or naked lady, is a flower that resembles the true crocuses, but blooms in autumn. The name “naked lady” comes from the fact that the flowers emerge from the ground long after the leaves have died back. Photo Attriubtion: Creative commons
2015 Copyright. Holistic Oncology Movie and agents. All rights reserved.
Disclaimer. This is an educational site and nothing therein can be construed to be medical or legal advice. This pink naked lady flower can be quite poisonous if ingested and should only be visually and olfactorally appreciated.
 TOP: Scientific Name: Colchicum autamanale.Other Common Names: Meadow Saffron, Autumn Crocus, Naked Lady. Pharmaceutically, the bulb-like corms of Colchicum autumnale (crocus) contain colchicine, a molecule that has a narrow therapeutic index (ie, the US FDA and the European EMA have only approved it for the treatment of gout and familial Mediterranean fever). Research and clinical trials are examaning its usefulness for cancer control and reversal.
TOP: Scientific Name: Colchicum autamanale.Other Common Names: Meadow Saffron, Autumn Crocus, Naked Lady. Pharmaceutically, the bulb-like corms of Colchicum autumnale (crocus) contain colchicine, a molecule that has a narrow therapeutic index (ie, the US FDA and the European EMA have only approved it for the treatment of gout and familial Mediterranean fever). Research and clinical trials are examaning its usefulness for cancer control and reversal.![]()
Leave a Reply